View all Courses
Research Ethics
There are two levels of Research Ethics Committees in the Institute, at Faculty/Department level and at Institute level (IREC).
All staff and students of ATU Sligo are required to plan and conduct their research investigations in accordance with appropriate ethical standards. Staff should ensure that they have knowledge of any relevant disciplinary guidelines on research ethics and that any empirical research has the required approval by the Research Ethics Committees.
All potential applicants should be familiar with the Research Ethics Policy and the related Research Ethics Procedure.
The function of each of the Research Ethics Committees is to safeguard the health, welfare and rights of human participants and researchers in research studies. For any research proposal to gain ethical approval it must be necessary and of a design that minimises predictable risk to both the research participant and the researcher.
The Research Ethics Committees aspires to provide comprehensive and independent reviews of the ethics of proposed studies, acting in accordance with good ethical practice as dictated by relevant EU Directives, National legislation and practice guidelines. If the proposed research involves patients (i.e. people who are receiving treatment as a result of an illness) the applicant should seek confirmation of the need to submit their application to the relevant hospital’s Research Ethics Committees.
Before Applying
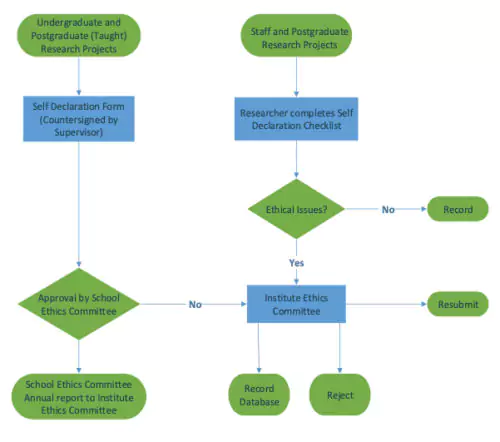
Applicants should consider which research ethics committee that their application needs to be considered at by reviewing this flowchart.
The IREC only reviews applications from staff research projects and post graduate research students and those applications which the Schools Research Ethics Committees feel require further consideration by the IREC.
The IREC does NOT consider applications from taught Masters Students or undergraduate students.
For School Approval, please see contact details below:
Faculty of Business & Social Sciences
ssrec.sligo@atu.ie
Faculty of Science
hrec.sligo@atu.ie
How to Apply?
Applications to Faculty/Department Research Ethics Committee:
Please liaise with your lecturer/ supervisor to ascertain the process for applying to your School/ Department research ethics committee.
Applications to Sligo Institute Research Ethics Committee:
Applicants are requested to complete a Research Ethics Approval Application Form.
Please email your application to both chris.omalley@atu.ie and emma.flanagan@atu.ie.
We will acknowledge receipt of your application by providing a Reference number to you by email.
The IREC recommends to view the videos below before making an application.
When and why ethical approval is needed
Where and how to apply for approval & Preparing for application
The form, data on participants, asking for consent, etc.
Advising participants on their involvement, inducements to participate
Participant information sheet, Consent form, Signature page, etc.
Engaging with Ethics Committee, Overview of process, Most common issues, etc.
Where the research involves interviewing or surveying people, the application also needs to be accompanied by:
- A copy of the Consent Form and
- A copy of the Participant Information Sheet (advising participants of what the research is for, how their role in it will work and what may/is to be done with the results) of the research and
- A copy of the Questionnaire or Interview Guidelines to be used
- Together with the applicant’s signature (Download Signature Page Template).
These documents should be emailed to the Chair of the Institute’s Research Ethics Committee at chris.omalley@atu.ie. and emma.flanagan@atu.ie.
In advance of the deadline for applications for the Committee meeting where you wish the application to be considered.
Please note the applications will not be processed without the signature page. Signatures must be original.
In cases where other research ethics committees have reviewed the application, copies of their responses must be uploaded as part of the application process.
As soon as feasible after the closing date for receipt of applications, the application will be allocated an IREC reference number. This number will be sent to the principal investigator to acknowledge receipt of the application. This number should be quoted on any further correspondence between the IREC and the principal investigator.
IREC Committee Meeting & Submission dates September 24 – May 2025
Feedback will be provided by the committee in a constructive capacity that is intended to support the applicants in their research.
| IREC Committee Meeting Date | Deadline for Receipt of Application |
|---|---|
| Monday 7th October 2024 | Friday 27th September 2024 |
| Wednesday 27th November 2024 | Monday 18th November 2024 |
| Wednesday 29th January 2025 | Monday 20th January 2025 |
| Monday 3rd March 2025 | Friday 21st February 2025 |
| Wednesday 30th April 2025 | Monday 21st April 2025 |
| Monday 26th May 2025 | Friday 16th May 2025 |
Helpful Links and Documents
National and international guidance documents
- International Ethical Guidelines for epidemiological studies WHO
- Operational Procedures for research ethics committees
- HRB policy of research ethics
- Ethics for researchers EUROPE
- inf206 guidelines for internet mediated research
Animal / Genetic research
- HRB Paper on the Legal and Ethical Considerations for Genetic Research
- HRB Policy on use of animals in research
Data Protection
- Webinar-on-GDPR-and-health-research-regulations-2018
- Data protection guidelines on research in the health sector
- Data Protection Act 2003
Vulnerable groups

